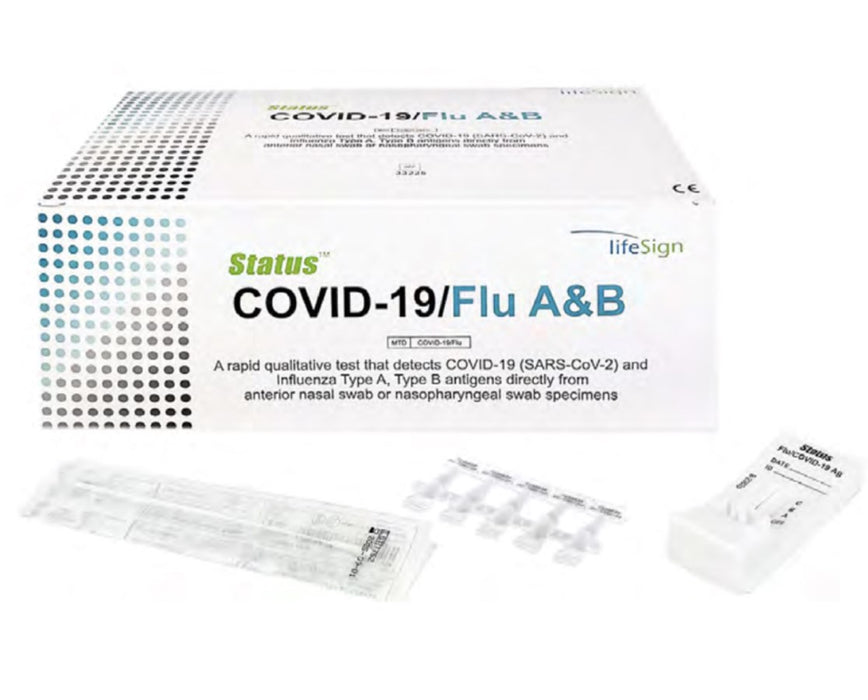
Status COVID-19/Flu A&B Test Kit (25/bx)
The cart will thereforce automatically set Qty to 1 when you order this product
This product is only allowed to a Medical Professional
Automatic Discount for ADC, DYNAREX, RIESTER & SECA products when you purchase 2+
WE CARRY QUICK-SHIP ADIRMED FURNITURE ORDER NOW >


The cart will thereforce automatically set Qty to 1 when you order this product
Deliver every:
This product is only allowed to a Medical Professional
The LifeSign Status COVID-19/Flu A&B Test Kit is a rapid, easy-to-use immunoassay that detects and differentiates SARS-CoV-2, Influenza A, and Influenza B. This helps clinicians pinpoint the actual cause of seemingly similar symptoms, enabling fast, accurate diagnostic decisions at the point of care.
✔ COVID-19 PERFORMANCE: Anterior Nasal 93.8% / 100% | Nasopharyngeal 93.1% / 100% (Sensitivity / Specificity).
✔ FLU PERFORMANCE: Flu A: 91.4% / 95.7% | Flu B: 87.6% / 95.9% (Sensitivity / Specificity).
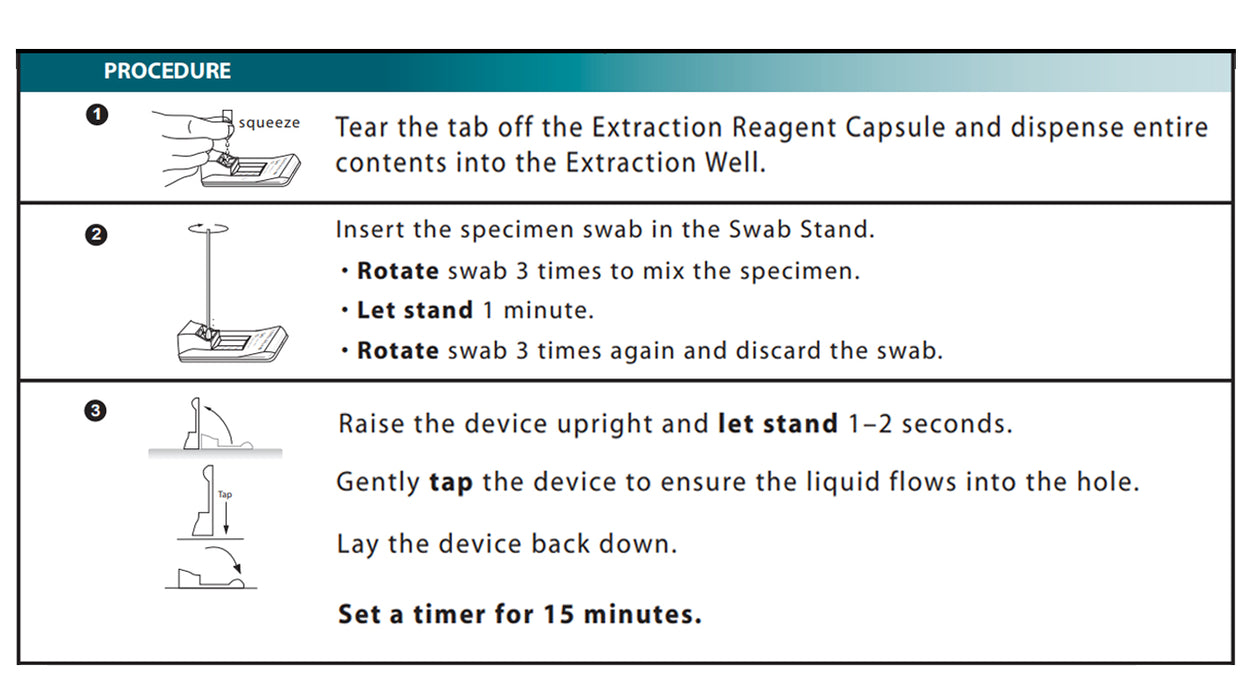
✔ FLEXIBLE SPECIMEN COLLECTION: Specimen is collected through anterior nasal or nasopharyngeal swabbing, within the first 5 days of symptom onset. Uses soft flocked swabs to minimize discomfort during specimen collection.
✔ CLIA-WAIVED & FDA EUA AUTHORIZED: Approved for use in patient care settings under CLIA Waiver, Certificate of Compliance, or Accreditation.
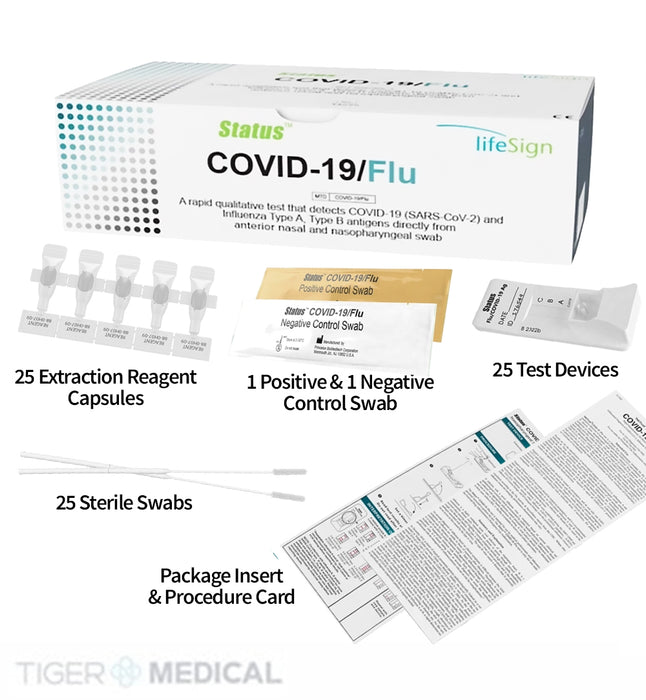
✔ COMPLETE KIT INCLUDED: Comes with flocked swabs, extraction reagents, test devices, and controls for immediate setup.
Engineered for clinical precision, the Status™ COVID-19/Flu A&B Test Kit empowers healthcare providers to diagnose respiratory infections accurately and efficiently, delivering fast answers when every minute matters.
![]() WARNING: This product can expose you to chemicals known to the State of California to cause cancer. For more information, go to www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals known to the State of California to cause cancer. For more information, go to www.P65Warnings.ca.gov.
ITEMS WITH A LEAD TIME LONGER THAN 48 HOURS CANNOT BE RETURNED / CANCELLED. These are specially ordered, and we cannot accept returns or cancellations on such products for any reason. We take the responsibility to make sure your product arrives in brand new working condition. Should your item arrive damaged, we will replace the product at no cost to you.
ITEMS WHICH SHIP OUT IN 24-48 HOURS. These are covered by our standard 30 day return policy from date of shipment. Return shipping charges are the responsibility of the customer. The product must be in new condition and in its original packaging.
For complete details of our return policy and process, please refer to our general return policy page.
ALLOCATED PRODUCTS BELOW ARE NON-CANCELLABLE, NON-RETURNABLE
{"one"=>"Select 2 or 3 items to compare", "other"=>"{{ count }} of 3 items selected"}



